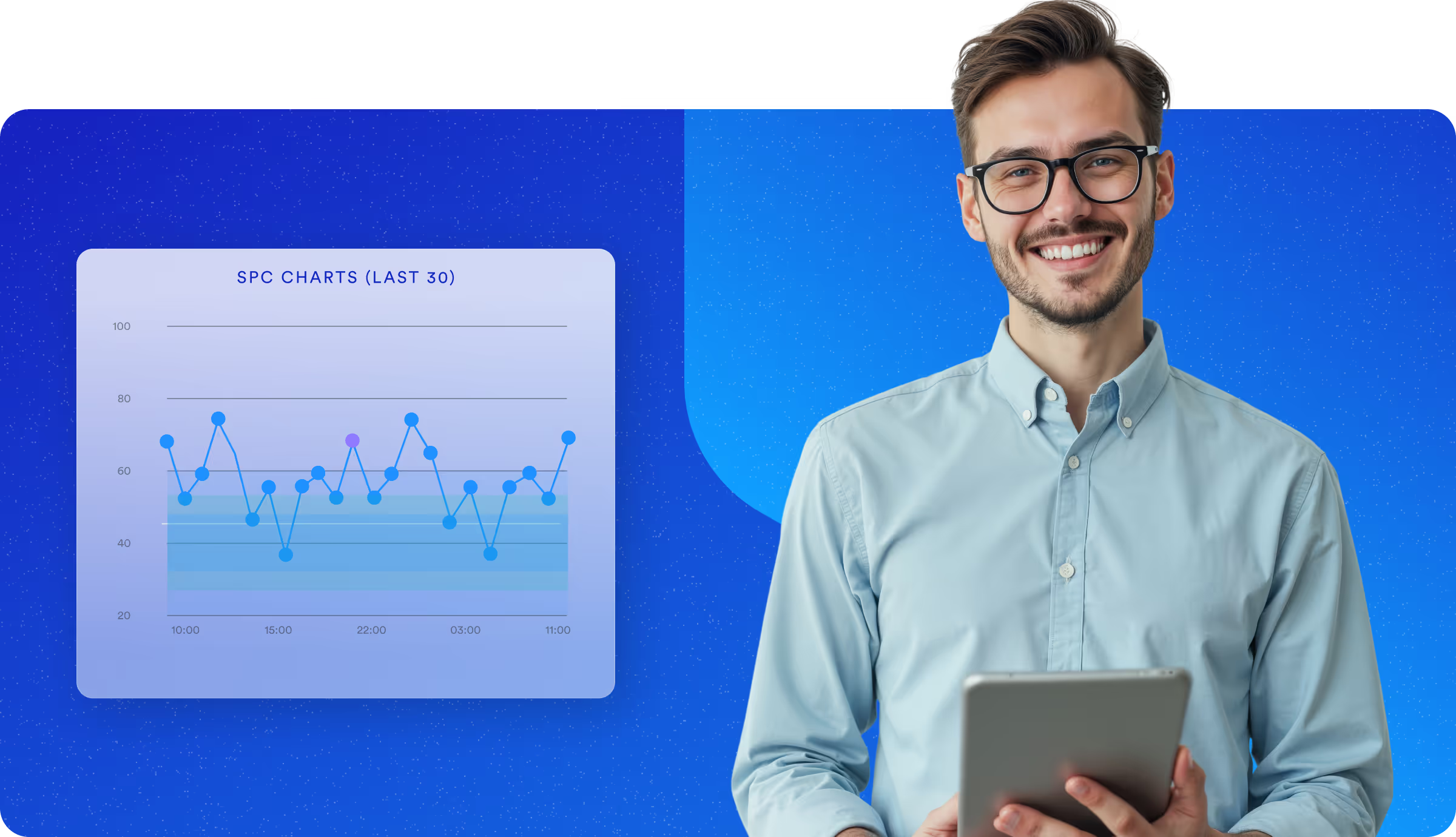
Built for the Highest Standards of Quality and Compliance
Life sciences manufacturers operate under rigorous regulations and tight tolerances. TrakSYS delivers digital traceability, real-time quality enforcement, and audit-ready records, ensuring you meet regulatory requirements without slowing down innovation.
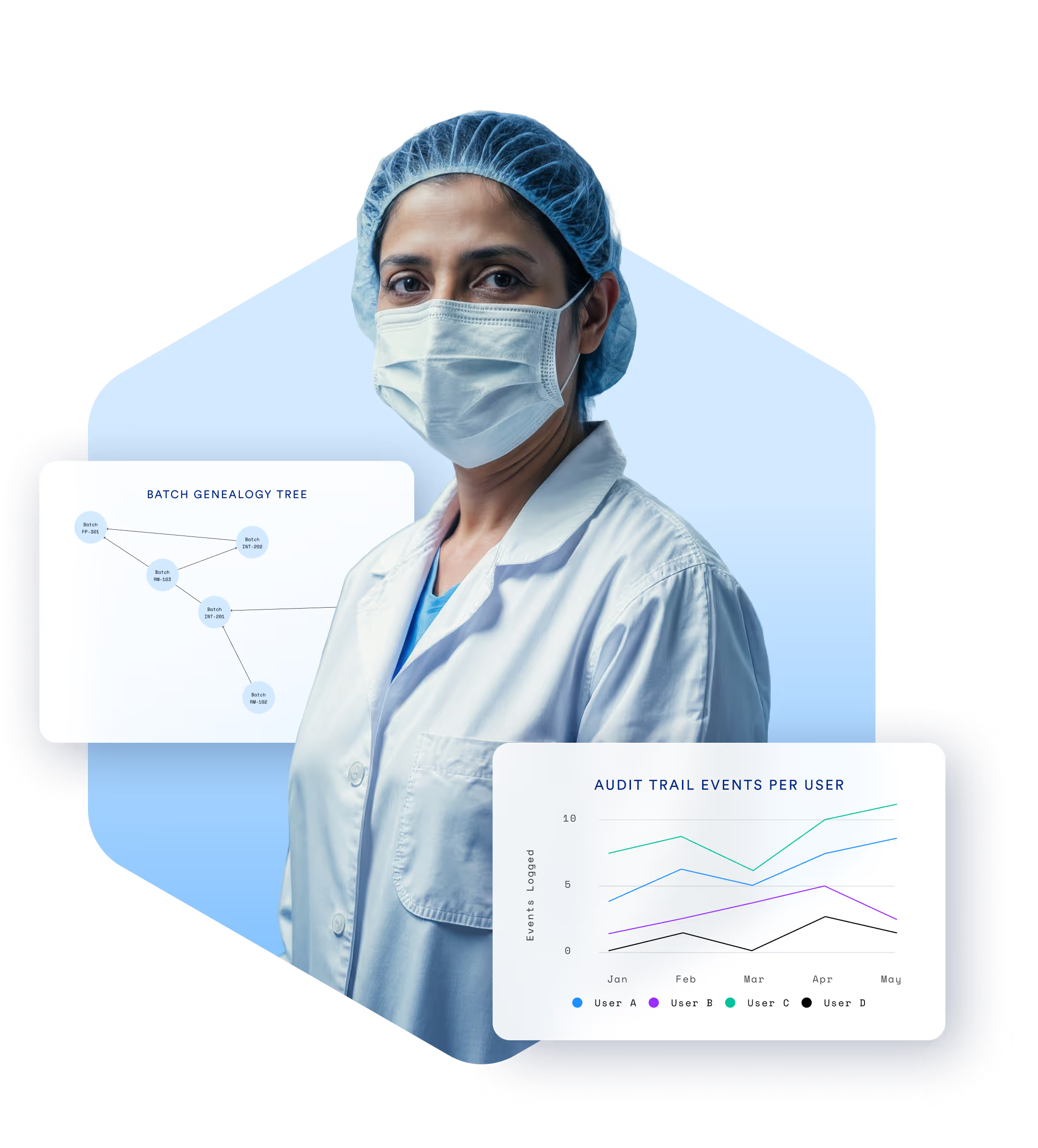
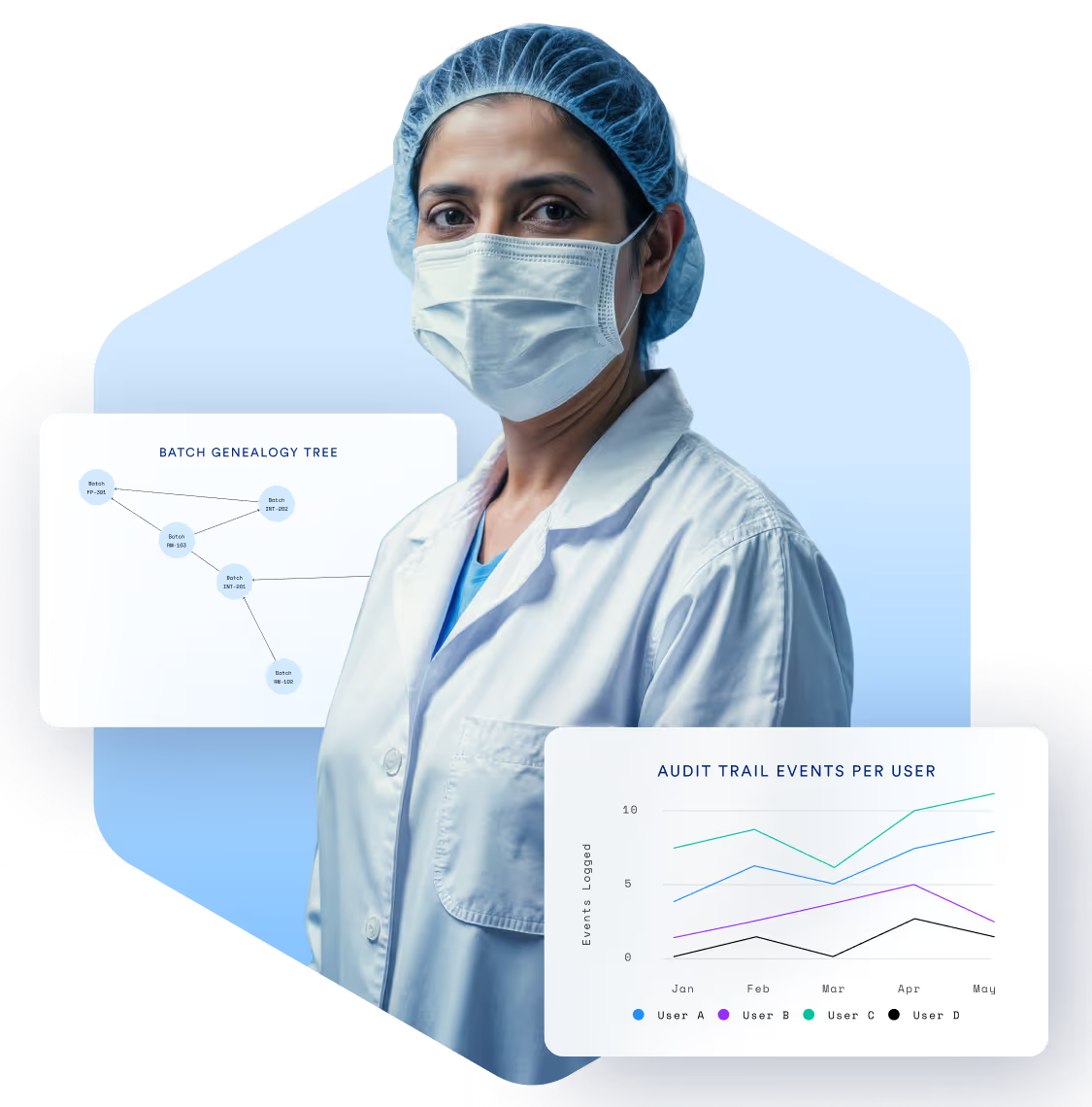
Compliance Isn’t
Optional. It’s Essential.
Manufacturers in pharma, biotech, and medical devices must meet the highest standards of quality, documentation, and traceability. TrakSYS helps you enforce SOPs, capture digital batch records, and respond quickly to audits—all while supporting continuous improvement.
Enforce GMP workflows with digital records.
Monitor quality in real time, with no delays.
Enable end-to-end traceability of materials, batches, and products.
Generate audit-ready reports at the click of a button.


TrakSYS gives you the digital backbone to support your GMP compliance goals while optimizing operations.


What You Get
with TrakSYS
Digital Batch Records
Log every step of your production process—seamlessly.
Deviation & CAPA Management
Track and resolve non-conformances to stay audit-ready.
Regulatory Compliance Support
Meet FDA, EMA, and GMP requirements with confidence.
Audit-Ready Reporting
Instant access to quality and compliance records for inspections.


Real-World Results:
A Life Sciences Success Story
Discover how TrakSYS helped AstraZeneca digitize batch records, enforce quality checks, and maintain audit readiness—all while empowering operators and improving efficiency.
TrakSYS Powers Life
Sciences Excellence
Leading life sciences manufacturers trust TrakSYS to meet regulatory requirements, ensure product quality, and drive continuous improvement all from a single, secure platform.