Simplifying EBR with Digital Batch Management in TrakSYS
In this webinar, Parsec’s Nic Azad is joined by Dave Ray, Director of Product Advancement, and Christopher Gray, Senior Applications Engineer, to explore how TrakSYS simplifies Electronic Batch Records (EBR) through Digital Batch Management.
Together, they discuss how manufacturers can move beyond paper-based systems to achieve higher accuracy, speed, and compliance without disrupting production. The conversation covers common challenges—such as complex batch processes, manual data collection, and regulatory requirements—and how TrakSYS overcomes them through automation, connectivity, and workflow intelligence.
Key topics covered include:
- How EBR and Digital Batch Management drive faster, more accurate operations
- Common implementation challenges and how TrakSYS helps overcome them
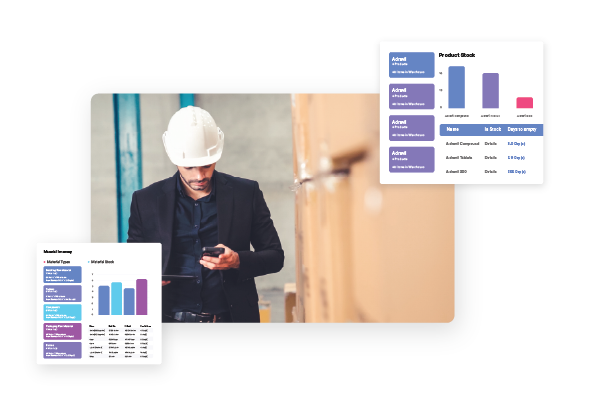
- Native connectivity to automation, ERP, and quality systems for contextualized data
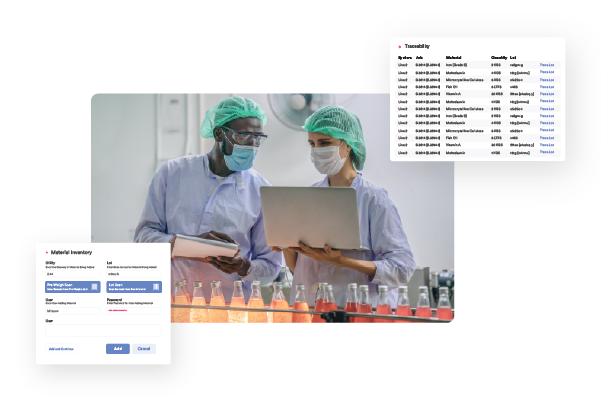
- Workflow automation for deviations, approvals, and digital signatures
- Real-world demonstration of batch management for both manual and automated processes
- Compliance support for FDA 21 CFR Part 11 and digital audit trails
With TrakSYS, manufacturers can unify batch control, compliance, and visibility in one modular MOM platform—accelerating digital transformation across regulated and nonregulated environments.
Related Videos


What are You Working on Right Now?
Performance? Quality? E-Records? Tell us what you’re working on. Chances are, we can help.